Digital transformation is growing rapidly year-on-year in the healthcare industry. Spending on digital transformation surpassed $1.3 trillion worldwide and it is growing at a whopping 10.4% year on year. In a study conducted by Deloitte, around 92% of healthcare professionals and institutes achieved better performance from digital transformation [1]
In an era defined by rapid technological advancements, digital transformation is transforming the landscape of the pharmaceutical and healthcare industry. With the convergence of cutting-edge technologies and data-driven approaches, the industry is witnessing a remarkable shift towards enhanced patient care, streamlined operations, and ground breaking innovations.
From leveraging artificial intelligence and machine learning for drug discovery to implementing tele-health solutions for remote patient monitoring, digital transformation is paving the way for a future where healthcare is more accessible, efficient, and personalised. This transformative journey holds the promise of improving patient outcomes, optimising processes, and unlocking new frontiers in medical research, ultimately shaping the future of healthcare delivery and empowering both patients and practitioners with unprecedented opportunities for better health and well-being.
How Can Data Science Aid Life Sciences in This Digital Transformation Era?
In an article titled, ‘Creating benefits by digitalisation for the bioprocess industry – The importance of Data Science as central enabler’, the author discusses how digitalisation and the Internet of Things (IoT) are transforming the bioprocess industry under the buzzword, Industry 4.0. Digitalisation creates new forms of innovation and results in new business models for the bioprocess industry. It will have an impact throughout its value chains, from logistics to marketing/sales and supplier/customer integration.
Digitalisation for the bioprocess industry will mainly act on two dimensions:
- The process chain – the enhancement of the process chain by digitalisation will act on the supply chain, logistics, and predictive maintenance
- The product life cycle – Digitalisation for the bioprocess industry covers the complete product lifecycle and will lead to flexibilisation of production by analysing sales and basing quick product changeovers on platform knowledge
The strategy to find a sustainable solution for digitalisation for the bioprocess industry is knowledge. There is a strong need for generic software tools to generate knowledge [2].
The author also discusses key enablers for digital transformation of the bioprocess industry, and that is Data Science.
Enabler 1: Real Time Environment
Enabler 2: Data Management System
Both enablers can be portrayed by the visual below:
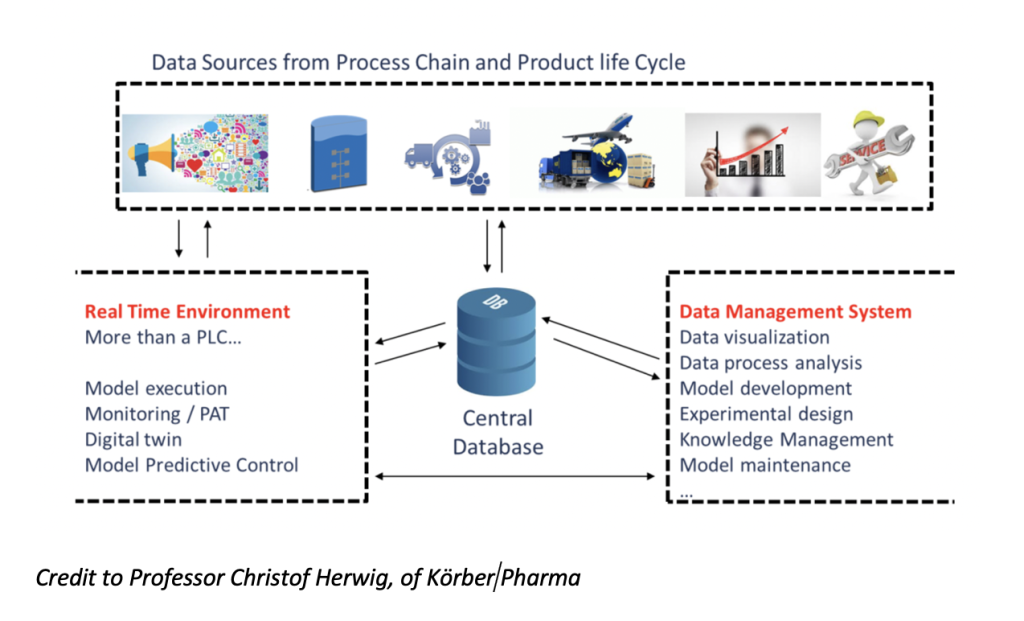
Where Can These Data Science Enablers Add Value?
Enabler 1 – Model Predictive Control from the ‘Real Time Environment’
To expand on the above article, we can discuss Model Predictive Control from the ‘Real Time Environment’ Data Science enabler. We can consider this a better option than preventative maintenance for many reasons:
Cost Efficiency: Predictive control focuses on identifying potential issues or failures before they occur by analysing real-time data from various sources. This approach allows for more targeted maintenance actions, reducing unnecessary maintenance and associated costs. Preventative maintenance, on the other hand, follows predefined schedules and may lead to unnecessary maintenance activities or parts replacement, which can be costly.
Data-Driven Decision Making: Predictive control relies on data analytics and advanced algorithms to drive maintenance decisions. It leverages real-time data, historical records, and machine learning techniques to identify patterns, predict failures, and optimise maintenance activities. This data-driven approach allows for more accurate and informed decision making compared to a fixed preventative maintenance schedule.
We haven’t even touched on the attributes; minimising downtime, increased equipment lifespan, and enhanced safety and quality.
Example of a ‘Real Time Environment’ Data Science Enabler – Model Predictive Control
We could potentially develop a Decision Support System (DSS) to predict failures from a peristaltic pump, during the ‘fill finish’ stage, of the Biopharma workflow using a Bayesian Network. And integrate this with a digital twin, to make analysis in real time. As fill finish is the most critical stage of the Biopharma workflow, it’s important to have our DSS in place here. Background knowledge is critical and knowledge acquisition can be performed to determine risk factors for key stakeholders. It can be obtained from specific sources. However, in this case, machine health v time to failure graphs and calculating conditional probability distributions (CPDs) are key. After obtaining the CPDs, we can construct our DSS with CPDs assigned to each node.
By querying the Decision Support System (DSS) and posing questions to the network, our stakeholders can estimate risks and identify potential delays. This proactive approach enables stakeholders to anticipate issues and mitigate them in advance, rather than responding reactively.
Enabler 2 – Model Development from the ‘Data Management System’
Additionally, we can discuss model development from the ‘Data Management System’ Data Science enabler. Model development enables pharmaceutical companies to leverage data-driven approaches, improve decision-making, accelerate drug development, enhance patient care, and optimize various aspects of their operations. It has the potential to transform the pharmaceutical industry by advancing scientific discoveries, improving patient outcomes, and driving innovation.
Example of a ‘Data Management System’ Data Science Enabler – Model Development
Human error poses a significant risk to product quality and implies negative cost deviations. Harry [3] estimates that the cost of the pharmaceutical and biotech industry’s average cost deviation following human error ranges from $25,000 to $55,000. Nonetheless, the quality by design (QBD) concept presents a viable solution to quality check and delay challenges resulting from unpredictable human behaviour in manufacturing processes. As a risk management concept, QBD, especially in pharmaceutical batch processing, aims at bridging the gap between cost-cutting and product quality.
Therefore, we could build a model to predict the success of a manufacturing batch. Currently, subject matter experts in the pharmaceutical and biotechnology industry make the decisions. However, this Machine Learning (ML) model could potentially make decisions and predictions based on the success of these manufacturing batches. The projections or decisions relate to the calibre of critical raw materials as they correlate with critical process parameters (CPPs) along with final products critical quality attributes (CQAs). Hence, all future decisions could become fully automated i.e data driven with ML, as opposed to manual interventions or decisions from the SME.
In today’s Pharma 4.0, a holistic control strategy in the pharmaceutical product life cycle, is undoubtedly the ideal strategy for optimising end-product quality while significantly minimising overhead costs along the product life cycle. ICH Q10, ‘Pharmaceutical Quality System’ and ICH Q12‘, ‘Product Lifecycle Management’ play a significant role here.
The business outcome is potential savings in human error. This would also lead to producing more efficient batches, faster process times, and increased revenues [4].
Of course, these two examples above are part of the proposed continuous improvement practices.
Could the Unified Namespace Play a Key Role in Our Digital Transformation Workflow?
It’s also important to mention the Unified Namespace (UNS), another proposed digital solution or strategic architectural approach within the digital workflow.
It provides a standardised approach to data sharing and interoperability between different systems and applications. With the explosive growth of the Industrial Internet of Things (IIoT) and Industry 4.0, the need for seamless integration and connectivity across disparate systems has never been more important. Will big Biotech adopt it though?
It’s the structure of our business data, all stored in one central location. A digital twin of the entire business. Hence, it simulates all equipment on plant, in real time. It has a file share structure, and we can add and remove nodes as we wish.
We can treat every piece of equipment on the plant and the enterprise system as a node. For instance, if we were introducing new software using our predecessor enterprise system, we would have to build discrete (disparate) connections. Or, build a new database to collect the relevant information from the relevant nodes, for the software to consume it.
However, with Industry 4.0 and the UNS, we only have to connect to this UNS via an IOT architecture/ 4.0 communication method. The protocol can point the software to the information it needs to consume. This unified name space gets us one step closer to our business processes, being a closed loop [5].
In essence, some benefits from the UNS for a proposed Pharma 4.0 plant include:
- Simple integration
- Scalability
- Improved data quality (with standard presentation and format) and security
Takeaway
The Covid19 pandemic acted as a springboard for many healthcare companies, accelerating the digital transformation process. It forced biopharma companies to prioritize investments in digital innovation, resulting in radical changes in how they conduct operations.
Some healthcare companies are ploughing ahead at great speed, such as Sanofi with their company-wide AI system called “plai”[6] and GSK with their AI innovation hub, AI/ ML fellowships and challenges [7]. For many others, it may take longer.
Digital transformation is an organisational shift, a revised mind set and a continuous work in progress. As opposed to a fixed term project. Can we really afford to be doing it the old way?
References:
[1] How digital transformation is driving action in healthcare
https://www.weforum.org/agenda/2022/09/health-information-system-digital-transformation-healthcare/
[2] Creating benefits by digitalisation for the bioprocess industry – The importance of Data Science as central enabler
https://www.koerber-pharma.com/blog/creating-benefits-by-digitalization-for-the-bioprocess-industry-the-importance-of-data-science-as-central-enabler
[3] Reducing Human Error In GMP Pharma/Biopharma Operations
https://www.pharmaceuticalonline.com/doc/reducing-human-error-in-gmp- pharma-biopharma-operations-0001.
[4] Malcolm, S. (2022). Binary Pass/ Fail Classification of Pharmaceutical Product Batches Based on Critical Raw Material Batch Properties, Critical Quality Attributes, and their Corresponding Critical Process Parameters [Manuscript in preparation]
[5] UNS Handbook — Introduction
https://www.linkedin.com/pulse/uns-handbook-introduction-walker-reynolds-1eqoc/
[6] Sanofi Goes All-In On AI
https://www.forbes.com/sites/alexzhavoronkov/2023/06/21/sanofi-goes-all-in-on-ai/amp/
[7] GSK uses AI to discover transformational medicines
https://www.gsk.ai/

